How AI is Expanding RWD for Clinical Trial Recruitment
The LIfe Sciences industry is experiencing a growing and signficant challenge in effectively executing clincial trials. Patient recruitment efforts are at an all time high, yet we’re seeing increasing rates of failure-to-launch due to insufficient patients. Over 80%, 4 out of every 5, clinical trial attempts fails due to insifficient patients. Further, greater than half of all global clinical trials fail due to inadeuqate patient recruitment or retention.
Fortunately, there are new tools to help the industry tackle this change. AI Large Language Models (LLMs) are enabling us to bring together insights from RWD sources, patient data sources, and public data sources to trinagulate the right patients for the right clinical trials.
Read on to see the way the industry is tackling these problems wiht novel usage of AI.
CONTENTS
-
What are the current trends in patient recruitment for clinical trials?
What are the common challenges being faced by the Life Sciences industry?
How can AI help address these needs?
-
How is AI is being used to identify the right patients for a given trial?
How can we generate new insights for better targeting of patients?
How can AI help better align patients to the right trial?
-
Evaluate the ways that AI can facilitate prioritizing identified patients to optimize the rates of participation and trial success.
How can AI improve retention of patients?
-
AI can improve assignment of patients to clinical trials with maximum chance of success.
How can AI facilitate the right site selection based on the right patient population?
How can AI be used to support recruitment for Decentralized and Virtual Clinical Trials?
-
What are the risks and concerns that must be addressed when leveraging AI for recruitment?
What are best practices for maintaining and operating AI programs?
-
Read the summary of the ways that AI can revolutionize patient recruitment, improving long-term success of clinical trials.
INTRODUCTION
Randomized Clinical Trials (RCTs, Clinical Trials) are the backbone of pharmaceutical innovation, driving the engine of development of new safe and efficacious drugs. However, we all know that this is an expensive, timely, and risk-intensive process; confounding attempts to continually speed up and improve the Clinical Trial process.
Patient Recruitment, the process of identifying, prioritizing, and recruiting the right patients, is one of the most significant bottlenecks in drug development. Studies show that over 80% of clinical trials fail to meet recruitment targets,i which dramatically impacts Clinical Trial success.
This has led to 4 out of 5 clinical trials failing to meet accrual targets, despite nearly $1.9 billion being spent on recruitment annually . Furthermore, serious concerns are being raised about the lack of diversity in study populations, which is especially critical in rare and orphan diseases. Significant impacts on studies include trial delays, increased costs, reduced analytical capabilities, limited statistical accuracy, and ultimately compromised clinical trial quality.iii We must identify new methods to optimize trial design for recruitment, feasibility, and inclusiveness.
Artificial Intelligence (AI) is emerging as a game-changer in patient recruitment. Given the ability to process Real World Data (RWD) and other datasets rapidly, efficiently, and at scale, AI can deliver faster and more extensive data-driven insights. AI can streamline patient recruitment, identifying eligible patients, prioritizing those most suitable for specific trials, and even enhance recruitment efforts, ultimately making the process more efficient and cost-effective.
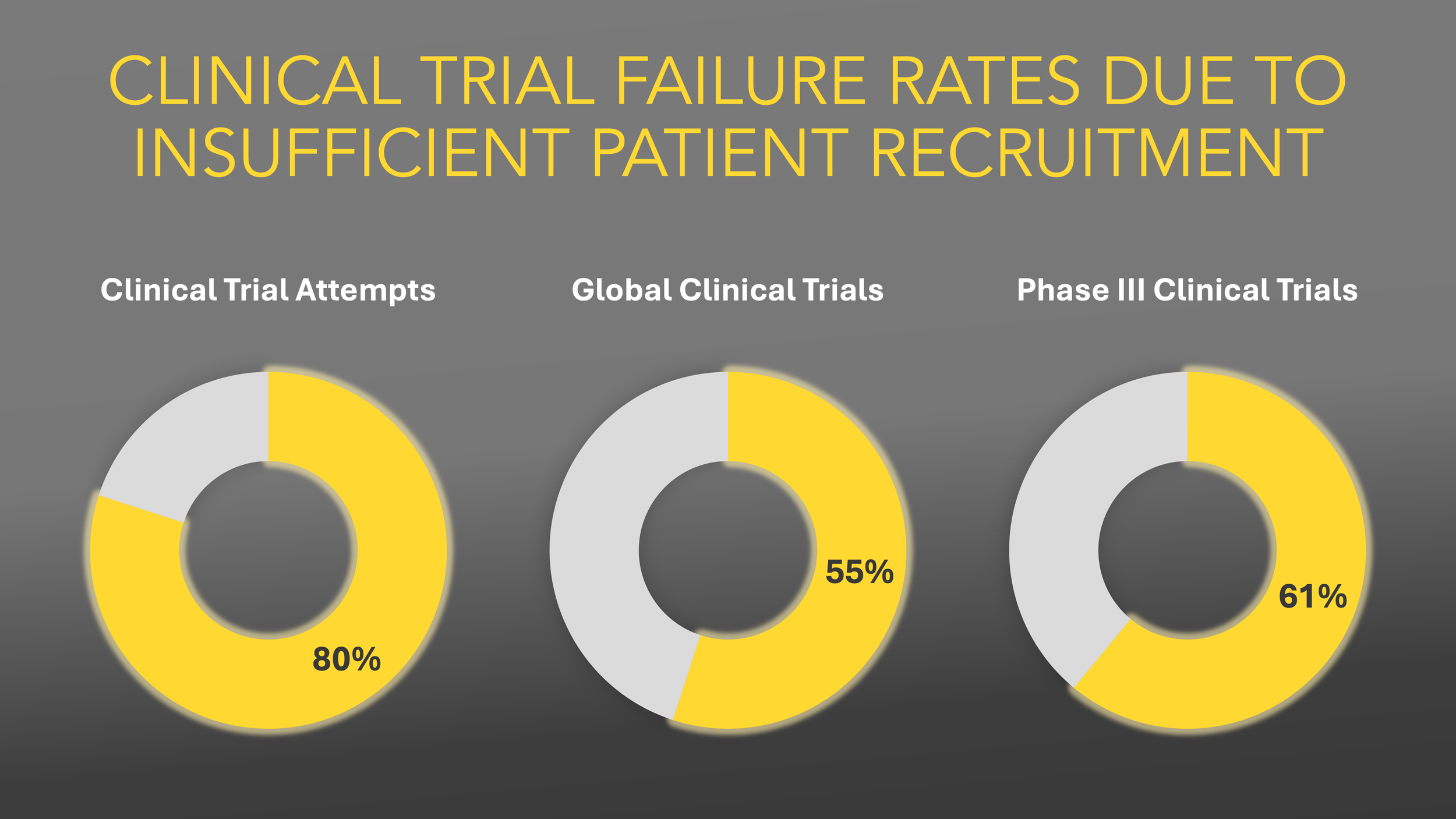
CLINICAL TRIAL FAILURE RATES
In the United States, more than 80% of clinical trials fail to achieve targeted patient enrollment, and 30% of study participants discontinue participation.
Globally 55% of clinical trials terminated due to low recruitment, with an average enrollment success rate of 40% for Phase III/IV trials.
1. IDENTIFYING ELIGIBLE PATIENTS WITH AI
To begin with, we must identify just who the right patients may be. Finding the right patients for a clinical trial requires analyzing and processing vast amounts of RWD, including electronic health records (EHRs), genetic profiles, and even patient-reported information. AI is simplifying this process by:
NATURAL LANGUAGE PROCESSING (NLP):
With a large majority of the data needed coming from EHRs, especially unstructured data, we need efficient ways to parse larger, more complex, and contextual volumes of data. AI-powered NLP can scan the complex unstructured data including medical notes, physician reports, and diagnostics to identify patients who meet clinical trial criteria. AI can extract key eligibility markers such as disease stage, medication history, and genetic predispositions.
Given that much of the critical data exists in these unstructured sources. Traditional patient screening requires manual review of medical records by analysts, a time-consuming and error-prone process. NLP automates this by extracting key eligibility criteria, including diagnoses, treatment history, biomarkers, comorbidities, and demographic details, from free-text.
Newer AI models have even been able to interpret contextual nuances, such as disease progression or medication adherence, ensuring more precise patient matching.
By integrating NLP with machine learning, pharmaceutical companies and research organizations can rapidly and efficiently identify eligible patients, reducing recruitment timelines and optimizing clinical trial enrollment.
RWE GENERATION
Amgen has used LLMs to extract RWE from unstructured clinical and patient data.
GENOMIC & BIOMARKER ANALYSIS:
GROWTH OF PRESONALIZED MEDICINE
Personalized medicine was $21.95B in 2024 and is projected to hit around USD 79.26 billion by 2034.
Precision medicine trials, targeting cellular-therapy and gene-therapy treatments are one of the fastest types of clinical trials. Personalized medicine was $21.95B in 2024 and is projected to hit around USD 79.26 billion by 2034.[i]
AI is particularly valuable where patient eligibility depends on very targeted genetic or biomarker profiles. These trials require extensive research, analysis, and recruitment efforts of broad populations just to identify the few potential candidates that may exist in any given location.
AI analyzes genetic and biomarker data to identify patients best suited for a given personalized clinical trial, particularly in precision medicine. AI can scan be directed to vast genomic datasets, identifying patients with specific mutations, gene expressions, or molecular markers that match trial eligibility criteria.
Additionally, AI has demonstrated the ability to conduct complex analysis across multi-omics data (genomics, proteomics, and metabolomics) to create more complex, complete, and accurate patient selection criteria.
This data-driven approach accelerates recruitment and enhances trial efficacy by ensuring that treatments are tested on the right patient populations, ultimately leading to more successful drug development and improved patient outcomes.
2. PRIORITIZING PATIENTS FOR CLINICAL TRIALS
Once AI identifies a pool of potential candidates, the next step is prioritizing patients based on suitability, willingness to participate, and likelihood of completing the trial. AI enhances this process by:
PREDICTIVE ANALYTICS:
AI can assess a patient’s likelihood of adhering to clinical trial protocols by analyzing a combination of medical history, behavioral patterns, and real-world data. Using predictive analytics and machine learning models, AI can evaluate factors such as past medication adherence, frequency of healthcare visits, socioeconomic conditions, and lifestyle habits to predict whether a patient is likely to follow trial requirements.
Additionally, as discussed earlier, AI-driven natural language processing (NLP) can be an invaluable asset to assessing patient data. AI can analyze patient interactions in healthcare records and online communities to gauge sentiment, motivation, and potential barriers to participation.
By identifying patients who are both eligible and likely to remain engaged throughout the trial, AI helps optimize recruitment strategies, reduce dropout rates, and improve overall trial success. This proactive approach ensures that clinical trials are conducted more efficiently, with higher-quality data and more reliable outcomes.
RISK STRATIFICATION:
AI ranks patients based on disease progression, comorbidities, and other risk factors, ensuring trials recruit individuals who are both eligible and likely to benefit from participation.
AI can enhance risk stratification in any given clinical trial by analyzing extensively more amounts of patient data to categorize participants based on numerous different data sources including disease severity, comorbidities, genetic predispositions, and potential treatment response.
AI can assess real-time and historical medical data, including EHRs, imaging scans, lab results, and biomarker profiles, to predict which patients may face higher risks of adverse events or disease progression. AI-driven predictive modeling enables researchers to balance trial cohorts effectively, ensuring a diverse yet medically stable participant pool.
Leveraging AI for risk stratification improves patient safety by identifying those who may need closer monitoring and enhances trial efficiency, leading to more reliable and generalizable study outcomes.
PERSONALIZED MATCHING:
AI enhances patient-centric recruitment by ensuring individuals are matched with trials that align with their medical history, preferences, and logistical constraints. This increases engagement and reduces dropout rates.
Personalized identification and matching of clinical trial candidates can be achieved through the effective combination of multiple AI capabilities. By leveraging machine learning, natural language processing (NLP), and predictive analytics to analyze vast datasets, including EHRs, genetic data, lifestyle factors, and patient preferences.
By integrating diverse data points, AI can match individuals to clinical trials based on their unique medical profiles, disease stage, biomarkers, and even likelihood of adherence. Unlike traditional recruitment methods that rely on broad eligibility criteria, AI-driven personalization ensures that each patient is matched to the most relevant and beneficial trial, increasing the chances of successful enrollment and retention.
This patient-centric approach not only enhances recruitment efficiency but also improves trial diversity and optimizes treatment outcomes, accelerating the development of precision medicine.
Example: Companies like IQVIA use AI-driven recruitment tools to quickly find eligible patients and match them to trials using their medical and demographic information. This reduces dropout rates and improves retention. iii
PATIENT RECRUITMENT
Companies like IQVIA use AI-driven recruitment tools to quickly find eligible patients and match them to trials using their medical and demographic information.
3. STREAMLINING RECRUITMENT WITH AI-POWERED TOOLS
Even when eligible and prioritized patients are identified, recruitment remains a challenge. Many potential participants never hear about clinical trials or are hesitant to enroll. AI helps bridge this gap through:
AUTOMATED OUTREACH:
AI can revolutionize automated outreach for clinical trial recruitment by using chatbots, predictive analytics, and personalized communication strategies to efficiently engage potential candidates. AI-driven systems can analyze patient data from EHRs, social media, or health forums to identify individuals who match trial criteria and then initiate targeted outreach through email, SMS, phone calls, or patient portals.
AI-powered tools can offer tailored trial information, respond to frequently asked patient questions, and assist candidates throughout the enrollment process, alleviating the burden on healthcare providers and the trial coordinators.
This streamlined, patient-friendly approach enhances trial accessibility, accelerates enrollment, and improves overall recruitment success.
AI-POWERED SITE SELECTION:
By analyzing claims, diagnostics, and EHR data, AI can identify patient distributions, helping pharmaceutical companies select optimal trial sites where recruitment success is more likely. This prevents trial locations from being underutilized or geographically misaligned with patient populations.
AI can significantly improve site selection for clinical trials by analyzing patient distribution data to identify optimal trial locations based on patient demographics, historical enrollment success, healthcare infrastructure, and investigator performance.
AI can additionally assess factors such as disease prevalence, local physician expertise, site efficiency, and regulatory environments to pinpoint locations where recruitment is most likely to succeed. Further, AI can evaluate past trial data to identify traditional bottlenecks, dropout rates, and site-specific challenges, allowing sponsors to make data-driven decisions that minimize delays and costs.
By ensuring trials are conducted in geographically and demographically diverse areas, AI-driven site selection enhances patient access, accelerates recruitment, and improves trial outcomes, ultimately leading to more successful and inclusive research.
VIRTUAL & DECENTRALIZED TRIALS:
AI enables remote patient monitoring and digital consent processes, allowing patients to participate in trials from home. This expands access to underrepresented populations and accelerates enrollment.
AI can enhance decentralized clinical trials (DCTs) by effectively expanding the rate and quality of remote patient monitoring, real-time data collection, and personalized engagement companies can execute. This has proven to ensure higher retention rates and improved outcomes.
By leveraging wearable devices, mobile health apps, and AI-driven analytics, researchers can continuously track patient vitals, medication adherence, and symptom progression without requiring frequent in-person visits. AI-powered chatbots and virtual assistants provide patients with real-time support, answering questions and reminding them of key trial milestones, reducing the risk of dropouts. Additionally, machine learning models can analyze vast datasets to detect early signs of adverse events, enabling proactive interventions.
This data-driven, patient-centric approach makes trials more accessible, especially for underrepresented populations, while ensuring high-quality data collection and more reliable study results.
DECENTRALIZED TRIALS
AI can enhance decentralized clinical trials (DCTs) by effectively expanding the rate and quality of remote patient monitoring, real-time data collection, and personalized engagement companies can execute
4. CHALLENGES & ETHICAL CONSIDERATIONS
Despite AI’s potential, there are challenges that pharmaceutical companies must address:
DATA PRIVACY & COMPLIANCE:
AI systems must comply with regulations like HIPAA and GDPR to ensure patient data is protected. This can create major blockers in progress towards leveraging AI. Ensuring AI models used for clinical trial recruitment are GDPR and HIPAA compliant require complex data privacy management processes built end-to-end in your AI model.
Pharmaceutical companies must implement robust data privacy and security measures throughout the AI lifecycle.
First, all patient data must be anonymized or pseudonymized to prevent direct identification, ensuring compliance with both regulations.
Second, AI systems should follow the principle of data minimization, collecting only the necessary patient information relevant to trial eligibility.
Third, organizations must obtain explicit patient consent before processing health data, ensuring transparency in how AI-driven recruitment operates.
Finally, regular compliance audits and regulatory reviews should be conducted to align AI practices with evolving data protection laws, ensuring ethical and legally sound recruitment processes.
BIAS & FAIRNESS IN AI MODELS:
AI models can inherit biases from historical data, leading to disparities in patient selection. Continuous model refinement and diverse datasets are crucial to ensuring fair recruitment practices.
To ensure AI models address issues with bias and fairness, AI models must leverage diverse, representative training datasets that include patients from different appropriate demographics, ethnicities, socioeconomic backgrounds, and geographic locations. Bias often arises when AI models are trained on historically skewed data, leading to the underrepresentation of certain populations in clinical trials. To mitigate this, companies may leverage AI models to also employ bias detection algorithms and conduct regular audits to identify and correct disparities in patient selection.
Additionally, implementing explainable AI (XAI) techniques ensures that recruitment decisions are transparent and interpretable, allowing researchers to verify fairness. Collaboration with ethics committees, regulators, and patient advocacy groups can further guide AI development to align with equitable recruitment practices.
Lastly, companies should continuously update and retrain AI models with fresh sources of Real-World Data (RWD) to prevent reinforcement of biases and ensure that all eligible patients have an equal opportunity to participate in clinical trials.
CONCLUSION
AI is revolutionizing clinical trial recruitment by enhancing patient identification, prioritization, and engagement. By leveraging machine learning, natural language processing, and predictive analytics, pharmaceutical manufacturers can reduce recruitment bottlenecks, improve trial efficiency, and ultimately bring life-saving treatments to market faster.
As AI continues to evolve, its role in clinical trials will only expand, making recruitment more accessible, equitable, and data-driven. Pharmaceutical companies that embrace AI in their recruitment strategies will gain a competitive edge in drug development and improve patient outcomes worldwide.
Are you ready to integrate AI into your clinical trial recruitment process? The future of efficient and effective trials is here—powered by AI.
REFERENCES
i Idnay B, Fang Y, Butler A, Moran J, Li Z, Lee J, Ta C, Liu C, Yuan C, Chen H, Stanley E, Hripcsak G, Larson E, Marder K, Chung W, Ruotolo B, Weng C. Uncovering key clinical trial features influencing recruitment. J Clin Transl Sci. 2023 Sep 4;7(1):e199. doi: 10.1017/cts.2023.623. PMID: 37830010; PMCID: PMC10565197. [Link]
ii Penberthy LT, Dahman BA, Petkov VI, DeShazo JP. Effort required in eligibility screening for clinical trials. J Oncol Pract. 2012;8(6):365–370. doi: 10.1200/JOP.2012.000646 [Link]
iii Campillo-Gimenez B, Buscail C, Zekri O, et al. Improving the pre-screening of eligible patients in order to increase enrollment in cancer clinical trials. Trials. 2015;16(1):1–10. doi: 10.1186/s13063-014-0535-7. [Link]
iv Personalized Medicine Biomarkers Market Size To Hit $79.26B by 2034 [Link]
v Patient Recruitment in Clinical Trials: Areas of Challenges and Success, a Practical Aspect at the Private Research Site [Link]
vi Accelerating Patient Identification through AI-driven Digital Solutions [Link]